阿地唑仑
外观
 | |
 | |
| 臨床資料 | |
|---|---|
| 给药途径 | 口服 |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 |
|
| 藥物動力學數據 | |
| 药物代谢 | 肝 |
| 生物半衰期 | <3小时 |
| 排泄途徑 | 肾 |
| 识别信息 | |
| |
| CAS号 | 37115-32-5 |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| 化学信息 | |
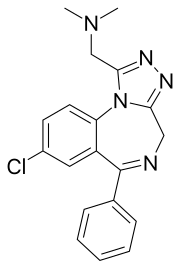
| 化学式 | C19H18ClN5 |
| 摩尔质量 | 351.8 |
| 3D模型(JSmol) | |
| |
| |
阿地唑仑(英语:Adinazolam[1]),商品名Deracyn,是三唑并苯二氮䓬类镇静剂,它是与三唑环融合的苯二氮䓬类。它具有抗焦虑[2]、抗惊厥、镇静和抗抑郁的特性。[3][4]阿地唑仑由杰克逊·海丝特开发,他正在寻求增强阿普唑仑(也是他开发的)的抗抑郁特性。[5]阿地唑仑从未获得FDA批准,也从未在公共市场上销售,但它已作为设计师药物出售。[6]
副作用
[编辑]过量使用该药物可能会造成包括肌肉无力、共济失调、构音障碍等副作用,尤其是儿童反常兴奋,以及在更严重的情况下可能会导致反射减弱、神经错乱和昏迷。[7]
一项比较阿地唑仑(30毫克和50毫克)与地西泮、劳拉西泮和安慰剂的主观影响和滥用可能性的人体研究表明,阿地那唑仑会导致最多的“精神和身体镇静”和最大的“精神不愉快”。[8]
阿地唑仑与外周型苯二氮䓬类受体结合,后者作为激动剂与GABA受体发生变构相互作用以产生抑制作用。
代谢
[编辑]在1984年8月出版的《药学与药理学杂志》中报道阿地那唑仑具有活性代谢物。[9]主要代谢物是N-去甲基阿地唑仑。[10]与其前体相比,N-去甲基阿地唑仑对苯二氮䓬受体的亲和力大约是其前体的25倍,这说明口服给药后会产生类似苯二氮䓬的作用。[11]多次N-脱烷基化导致二甲基氨基甲基侧链的去除,从而造成其效力的差异。[10]其他两种代谢物是α-羟基阿普唑仑和艾司唑仑。[12]在1986年8月的同一期刊中,塞西、弗朗西斯和戴依(Day)报道了普罗地芬抑制了N-去甲基阿地唑仑的形成。[13]
参见
[编辑]参考资料
[编辑]- ^ FR Patent 2248050
- ^ Karthik Venkatakrishnan; Lisa L. Von Moltke; Su Xiang Duan; Joseph C. Fleishaker; Richard I. Shader; David J. Greenblat. Kinetic Characterization and Identification of the Enzymes Responsible for the Hepatic Biotransformation of Adinazolam and N-Desmethyladinazolam in Man. Journal of Pharmacy and Pharmacology. March 1998, 50 (3): 265–274. PMID 9600717. S2CID 33656240. doi:10.1111/j.2042-7158.1998.tb06859.x.
- ^ Dunner D, Myers J, Khan A, Avery D, Ishiki D, Pyke R. Adinazolam-A New Antidepressant: Findings of a Placebo-Controlled, Double-Blind Study in Outpatients with Major Depression.. Journal of Clinical Psychopharmacology. June 1998, 7 (3): 170–172. PMID 3298327. doi:10.1097/00004714-198706000-00010.
- ^ Lahti, Robert A.; Vimala H. Sethy; Craig Barsuhn; Jackson B. Hester. Pharmacological profile of the antidepressant adinazolam, a triazolobenzodiazepine.. Neuropharmacology. November 1983, 22 (11): 1277–82. PMID 6320036. S2CID 667962. doi:10.1016/0028-3908(83)90200-9.
- ^ Discovers Award 2004 (PDF). Special Publications. Pharmaceutical Research and Manufacturers of America: 39. April 2004 [August 18, 2006]. (原始内容 (PDF)存档于August 24, 2006).
- ^ Moosmann, Bjoern; Bisel, Philippe; Franz, Florian; Huppertz, Laura M.; Auwärter, Volker. Characterization and in vitro phase I microsomal metabolism of designer benzodiazepines – an update comprising adinazolam, cloniprazepam, fonazepam, 3-hydroxyphenazepam, metizolam, and nitrazolam. Journal of Mass Spectrometry. 2016, 51 (11): 1080–1089. ISSN 1096-9888. PMID 27535017. doi:10.1002/jms.3840.
- ^ Adinazolam. DrugBank. [2023-02-10]. (原始内容存档于2020-02-12).
- ^ M. Bird; D. Katz; M. Orzack; L. Friedman; E. Dessain; B. Beake; J. McEachern; J. Cole. The Abuse Potential of Adinazolam: A Comparison with Diazepam, Lorazepam and Placebo (PDF). NIDA Research Monograph No. 81. 1987 [2015-12-17]. (原始内容 (PDF)存档于2016-12-22).
- ^ Sethy, Vimala H.; R. J. Collins; E. G. Daniels. Determination of biological activity of adinazolam and its metabolites.. Journal of Pharmacy and Pharmacology. August 1984, 36 (8): 546–8. PMID 6148400. S2CID 21094654. doi:10.1111/j.2042-7158.1984.tb04449.x.
- ^ 10.0 10.1 Peng, G. W. Assay of adinazolam in plasma by liquid chromatography. Journal of Pharmaceutical Sciences. August 1984, 73 (8): 1173–5. PMID 6491930. doi:10.1002/jps.2600730840.
- ^ 引用错误:没有为名为
FR2的参考文献提供内容 - ^ Fraser, A. D.; A. F. Isner; W. Bryan. Urinary screening for adinazolam and its major metabolites by the Emit d.a.u. and FPIA benzodiazepine assays with confirmation by HPLC. Journal of Analytical Toxicology. November–December 1993, 17 (7): 427–31. PMID 8309217. doi:10.1093/jat/17.7.427.
- ^ Sethy, Vimala H.; Jonathan W. Francis; J. S. Day. The effect of proadifen on the metabolism of adinazolam. Journal of Pharmacy and Pharmacology. August 1986, 38 (8): 631–2. PMID 2876087. S2CID 9394686. doi:10.1111/j.2042-7158.1986.tb03099.x.
