亚硝胺
外观
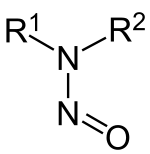
亚硝胺(英语:Nitrosamine)(不可称为亚硝酸胺)是一类通式为R1N(–R2)–N=O的胺化合物,其大部分成员都属强致癌物。
用途
[编辑]其中N,N'-二亚硝基五亚甲基四胺(N,N'-Dinitrosopentamethylenetetramine;发泡剂H)是一种重要的胶乳发泡剂;
环三亚甲基三亚硝胺(Trimethylenetrinitrosamine;R盐)为RDX的中间体之一,二战期间曾用作军用炸药。
分布
[编辑]亚硝胺在橡胶制品、食品和其他消费品中均有分布[1]。有研究预计避孕套中的亚硝胺没有毒理学意义。[2]
在酸性环境(如消化液)中,食物中的亚硝酸盐发生质子化而产生亚硝酰阳离子N≡O+与水:
- H
2NO+
2 → H2O + NO+.
N≡O+随即与胺(包括蛋白质及其降解产物)反应而生成亚硝胺[3] ;油煎、烧烤的高温对此特别有促进作用。
Liebermann反应能够提示亚硝胺的存在[4]。若检测结果呈阴性,则试剂显红色或兰色(苯酚存在时)。[5]
因亚硝化/亚硝解反应的存在,肉类、泡菜、奶酪等富含亚硝酸盐的食品中亚硝胺浓度水平极高。美国政府对肉制品亚硝酸盐含量实行严格规制,旨在降低公民罹患癌症的风险[6]。为抑制亚硝胺的生成,抗坏血酸或其衍生物被要求添加到肉制品中[7]。
包括鼻烟油、口含烟及嗅烟(尽管含量较前二者低)的烟草制品均含有亚硝胺[8]。电子烟生产中丙二醇的使用亦可引入痕量亚硝胺[9]。
致突变性
[编辑]动物实验已证实某些亚硝胺具有明显的致癌性,并提示其对人可能也有致癌作用。基于对照实验的流行病学调查表明,摄入亚硝胺与胃癌、食道癌的发生有著正相关性,但尚无证据直接证实亚硝胺的致癌性质。[10]
代表成员
[编辑]| 名称 | CAS号 | 别名 | 分子式 | 外观 | 致癌性分级 |
|---|---|---|---|---|---|
| N-亚硝基降烟碱 | 16543-55-8 | NNN | C9H11N3O | 淡黄色固体,熔点低 | |
| 4-甲基亚硝胺基-1-3-吡啶基-1-丁酮[11] | 64091-91-4 | NNK, 4′-(nitrosomethylamino)-1-(3-pyridyl)-1-butanone | C10H15N3O2 | 浅黄色油状液体 | |
| N-亚硝基二甲胺 | 62-75-9 | Dimethylnitrosamine, N,N-dimethylnitrosamine, NDMA, DMN | C2H6N2O | 黄色液体 | EPA-B2; IARC-2A; OSHA carcinogen; TLV-A3 |
| N-亚硝基二乙胺[12][13][14] | 55-18-5 | Diethylnitrosamide, diethylnitrosamine, N,N-diethylnitrosamine, N-ethyl-N-nitrosoethanamine, diethylnitrosamine, DANA, DENA, DEN, NDEA | C4H10N2O | 黄色液体 | EPA-B2; IARC-2A |
| 4-(甲基亚硝胺)-1-(3-吡啶基)-1-丙醇 | 76014-81-8 | NNAL | |||
| N-亚硝基新烟草碱 | 37620-20-5 | NAB | C10H13N3O | 黄色油状液体 | IARC-3 |
| N-亚硝基新烟碱 | 71267-22-6 | NAT | C10H11N3O | 黄色至橙色的清澈液体 | IARC-3 |
参见
[编辑]参考资料
[编辑]- ^ Altkofer, W; Braune, S; Ellendt, K; Kettl-Grömminger, M; Steiner, G. Migration of nitrosamines from rubber products--are balloons and condoms harmful to the human health?. Molecular nutrition & food research. 2005, 49 (3): 235–8. PMID 15672455. doi:10.1002/mnfr.200400050.
- ^ Proksch, E. Toxicological evaluation of nitrosamines in condoms. International journal of hygiene and environmental health. 2001, 204 (2–3): 103–10. PMID 11759152. doi:10.1078/1438-4639-00087.
- ^ Vogel, A. I. Practical Organic Chemistry 3rd. Impression. 1962: 1074.
- ^ Vogel, A. I. Practical Organic Chemistry 3rd. Impression. 1962: 649.
- ^ Glagovich, Neil. The Libermann Nitroso Reaction. Connecticut State University. [28 December 2015]. (原始内容存档于2013-03-05).
- ^ Honikel, Karl-Otto. The use and control of nitrate and nitrite for the processing of meat products. Meat Science. January–February 2008, 78: 68–76. doi:10.1016/j.meatsci.2007.05.030.
- ^ Scanlan, Dr. Richard A. Nitrosamines and Cancer. Nitrosamines and Cancer. The Linus Pauling Institute. 22 April 2000 [28 December 2015]. (原始内容存档于2015-12-28).
- ^ Gregory N. Connolly, and Howard Saxner. Informational Update Research on Tobacco Specific Nitrosamines (TSNAs) in Oral Snuff and a Request to Tobacco Manufacturers to Voluntarily Set Tolerance Limits For TSNAs in Oral Snuff. August 21, 2001.
- ^ E-cigarettes: harmless inhaled or exhaled, No second hand smoke. Health New Zealand. [27 June 2014]. (原始内容存档于2016-04-25).
- ^ Jakszyn, P; Gonzalez, CA. Nitrosamine and related food intake and gastric and oesophageal cancer risk: A systematic review of the epidemiological evidence. World journal of gastroenterology : WJG. 2006, 12 (27): 4296–303. PMC 4087738
 . PMID 16865769.
. PMID 16865769.
- ^ Hecht, Steven S.; Borukhova, Anna; Carmella, Steven G. "Tobacco specific nitrosamines" Chapter 7; of "Nicotine safety and toxicity" Society for Research on Nicotine and Tobacco; 1998 - 203 pages
- ^ [ NIH Substance Profile]
- ^ [ Spectrum; Chemical Fact Sheet]
- ^ [ Safety data for N-nitrosodiethylamine]
