Β-生育酚
外觀
| β-Tocopherol | |
|---|---|
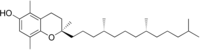
| |
| IUPAC名 (2R)-2,5,8-Trimethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydro-2H-1-benzopyran-6-ol | |
| 識別 | |
| CAS號 | 148-03-8 |
| PubChem | 86052 |
| SMILES |
|
| ChEBI | 47771 |
| 性質 | |
| 化學式 | C28H48O2 |
| 摩爾質量 | 416.68 g/mol g·mol⁻¹ |
| 沸點 | 200–210 °C(0.1 torr)[1] |
| 若非註明,所有數據均出自標準狀態(25 ℃,100 kPa)下。 | |
β-生育酚(英語:β-Tocopherol)是四種生育酚之一,分子式C28H48O2,是構成維生素E的化合物之一[2][3]。在氯化鎂、三乙胺存在下,它和甲醛反應,得到羥基鄰位甲酰化的產物;[4]它和四丁基三溴化銨反應,得到羥基鄰位溴化的產物;[5]和溴-氫氧化鈉反應時,則發生氧化-鹵化和重排反應。[6]
參考文獻
[編輯]- ^ "Drugs - Synonyms and Properties" data were obtained from Ashgate Publishing Co. (US). 2000. ISBN 0-566-08228-4. Retrieved from SciFinder. [2021-11-21].
- ^ Food and Agriculture Organization; World Health Organization. 9. Vitamin E. Joint FAO/WHO Expert Consultation on Human Vitamin and Mineral Requirements (報告). Bangkok, Thailand: FAO Rome. 2001 [2021-11-20]. (原始內容存檔於2022-04-01).
- ^ Burton, G. W.; Ingold, K. U. Autoxidation of biological molecules. 1. Antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro. Journal of the American Chemical Society. 1981, 103 (21): 6472–6477. doi:10.1021/ja00411a035.
- ^ Khaled Alsabil, Guillaume Viault, Sorphon Suor-Cherer, Jean-Jacques Helesbeux, Joumaa Merza, Vincent Dumontet, Luis Manuel Peña-Rodriguez, Pascal Richomme, Denis Séraphin. Efficient ortho-formylation in vitamin E series, application to the semi-synthesis of natural 5- and 7-formyl-δ-tocotrienols revealing an unprecedented 5-bromo-7-formyl exchange. Tetrahedron. 2017-12, 73 (49): 6863–6870 [2021-11-20]. doi:10.1016/j.tet.2017.10.039. (原始內容存檔於2018-06-15) (英語).
- ^ Jia-fei Poon, Vijay P. Singh, Jiajie Yan, Lars Engman. Regenerable Antioxidants-Introduction of Chalcogen Substituents into Tocopherols. Chemistry - A European Journal. 2015-02-02, 21 (6): 2447–2457 [2021-11-20]. doi:10.1002/chem.201405895 (英語).
- ^ Stefan Böhmdorfer, Anjan Patel, Andreas Hofinger, Thomas Netscher, Lars Gille, Thomas Rosenau. Bromination of Tocopherols: Oxidative Halogenations and Rearrangements. European Journal of Organic Chemistry. 2011-06, 2011 (16): 3036–3049 [2021-11-20]. doi:10.1002/ejoc.201100153 (英語).
外部連結
[編輯]- US Office of Dietary Supplements article on Vitamin E (頁面存檔備份,存於互聯網檔案館)
- Vitamin E risk assessment (頁面存檔備份,存於互聯網檔案館), Expert Group on Vitamins and Minerals, UK Food Standards Agency, 2003.
| ||||||||||||||||||||||||||||||||||||||||||||||||
| 這是一篇雜環化合物小作品。您可以透過編輯或修訂擴充其內容。 |
