5-羥色氨酸
外觀
| 5-羥色氨酸 | |
|---|---|

| |

| |
| IUPAC名 2-amino-3- (5-hydroxy-1H-indol-3-yl) propanoic acid | |
| 識別 | |
| CAS號 | 56-69-9 |
| PubChem | 144 |
| ChemSpider | 388413 |
| SMILES |
|
| InChI |
|
| InChIKey | LDCYZAJDBXYCGN-VIFPVBQEBZ |
| ChEBI | 17780 |
| KEGG | D07339 |
| MeSH | 5-Hydroxytryptophan |
| 性質 | |
| 化學式 | C11H12N2O3 |
| 摩爾質量 | 220.23 g/mol g·mol⁻¹ |
| 密度 | 1.484 g/mL |
| 熔點 | 298-300 °C(571-573 K) |
| 沸點 | 520.6 °C(794 K) |
| 若非註明,所有數據均出自標準狀態(25 ℃,100 kPa)下。 | |
5-羥色氨酸(英語:5-Hydroxytryptophan,簡稱5-HTP),也叫歐西曲坦(INN:oxitriptan),是一種天然的氨基酸代謝中間產物,同時也是血清素與褪黑素的生物合成前體。
5-羥色氨酸作為給抑鬱症、厭食症及失眠患者服用的膳食補充劑,在美國、英國和加拿大以非處方方式出售;同時也作為治療重性抑鬱障礙的藥物在歐洲許多國家銷售。[1][2]幾個安慰劑雙盲臨床試驗已證明5-HTP有治療抑鬱症的效果[1],但缺乏極顯著性[3],需要更進一步的臨床對照研究[4]。
代謝
[編輯]在神經組織和肝細胞中[5],5-羥色氨酸在芳香族L-氨基酸脫羧酶、維生素B6幫助下脫羧生成血清素(5-羥色胺)[6]。 5-羥色氨酸可以穿過血腦屏障[7],但血清素不能。過量的5-羥色氨酸會被代謝排出體外,體內的維生素B6會促進這種代謝。[8][9]
| 5-HTP | AAAD | 血清素 | |

|

| ||
| PLP | |||

| |||
藥理學
[編輯]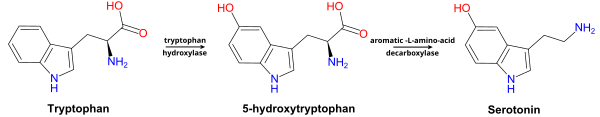
另見
[編輯]參考文獻
[編輯]- ^ 1.0 1.1 Turner EH, Blackwell AD. 5-Hydroxytryptophan plus SSRIs for interferon-induced depression: synergistic mechanisms for normalizing synaptic serotonin. Medical Hypotheses. 2005, 65 (1): 138–44 [2013-08-14]. PMID 15893130. doi:10.1016/j.mehy.2005.01.026. (原始內容存檔於2018-10-13).
- ^ Swiss Pharmaceutical Society. Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. 2000. ISBN 3-88763-075-0.
- ^ Shaw K, Turner J, Del Mar C. Shaw, Kelly A , 編. Tryptophan and 5-hydroxytryptophan for depression. Cochrane Database of Systematic Reviews (Online). 2002, (1): CD003198. PMID 11869656. doi:10.1002/14651858.CD003198.
- ^ 5-Hydroxytryptophan (5-HTP) (頁面存檔備份,存於網際網路檔案館) University of Maryland Medical Center. 2011. Accessed: 9 January 2012.
- ^ Bouchard, S; Bousquet, C; Roberge, AG. Characteristics of dihydroxyphenylalanine/5-hydroxytryptophan decarboxylase activity in brain and liver of cat. Journal of Neurochemistry. 1981, 37 (3): 781–7. PMID 6974228. doi:10.1111/j.1471-4159.1982.tb12555.x.
- ^ Rahman MK, Nagatsu T, Sakurai T, Hori S, Abe M, Matsuda M. Effect of pyridoxal phosphate deficiency on aromatic L-amino acid decarboxylase activity with L-DOPA and L-5-hydroxytryptophan as substrates in rats. Jpn. J. Pharmacol. 1982, 32 (5): 803–11. PMID 6983619. doi:10.1254/jjp.32.803.
- ^ Gomes P, Soares-da-Silva P. L-DOPA transport properties in an immortalised cell line of rat capillary cerebral endothelial cells, RBE 4. Brain Res. 1999, 829 (1–2): 143–150. PMID 18445233. doi:10.1016/S0006-8993(99)01387-6.
- ^ Bouchard S, Roberge AG. Biochemical properties and kinetic parameters of dihydroxyphenylalanine--5-hydroxytryptophan decarboxylase in brain, liver, and adrenals of cat. Can. J. Biochem. 1979, 57 (7): 1014–8. PMID 39668. doi:10.1139/o79-126.
- ^ Amamoto T, Sarai K. On the tryptophan-serotonin metabolism in manic-depressive disorders. Changes in plasma 5-HT and 5-HIAA levels and urinary 5-HIAA excretion following oral loading of L-5HTP in patients with depression. Hiroshima J. Med. Sci. 1976, 25 (2–3): 135–40. PMID 1088369.
- ^ 5-HTP: Uses, Side Effects, Interactions and Warnings - WebMD. [2009-10-05]. (原始內容存檔於2009-11-16).
延伸閱讀
[編輯]- den Boer JA, Westenberg HG. Behavioral, neuroendocrine, and biochemical effects of 5-hydroxytryptophan administration in panic disorder. Psychiatry Research. 1990, 31 (3): 267–78. PMID 2139731. doi:10.1016/0165-1781(90)90096-N.
- Angst J, Woggon B, Schoepf J. The treatment of depression with L-5-hydroxytryptophan versus imipramine. Results of two open and one double-blind study. Archiv für Psychiatrie und Nervenkrankheiten. 1977, 224 (2): 175–86. PMID 336002.
- article at Psychology Today
- Turner EH, Loftis JM, Blackwell AD. Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacol. Ther. 2006, 109 (3): 325–38. PMID 16023217. doi:10.1016/j.pharmthera.2005.06.004.
- 5-Hydroxytryptophan (5-HTP) Supplement Information(頁面存檔備份,存於網際網路檔案館) at University of Maryland Medical Center
